Long-time ALK survivor, ALK Positive, Inc. Board member, and prominent Medical Committee member, Jeff Sturm, writes about his personal experience participating in the University of Michigan’s ALK project by having his tumor tissue removed and tested to inform future treatment decisions.
My Biopsy and Organoid Drug Screening Experience with The UMichigan ALK NSCLC Initiative
I’m writing this on the return flight from the University of Michigan, where I underwent a thoracic biopsy for three lymph nodes that have been lighting up in my recent PET scans. As I am the ALK Positive patient advocate on the Judith Tam ALK NSCLC Research Initiative Scientific Advisory Board, l thought I’d do a trial run and see if going there for my biopsy and tissue testing would be worthwhile. This was an opportunity to donate tissue and blood to their program, to meet the scientists that are working hard for our benefit, and to take advantage of the drug screening ability they’ve developed with my own cancer tissue. Here’s the story.
I’ve had a few biopsies over nine years of ALK NSCLC treatment, and the thoracic procedures were rather uncomfortable, leaving me with a cough for weeks. Additionally, the thoracic surgeon we used previously in my local hospital has been unable to get enough tissue to do all the testing that Dr. Camidge, my second-opinion doctor, wanted for tests beyond NGS. Dr. Camidge told me I needed to use an interventional pulmonologist this time instead of a thoracic surgeon, and, while I could have gone to UCSF nearby for that, I chose a better option.
In talking with Dr. Angel Qin and Dr. Sofia Merajver at UMichigan, they offered their expert interventional pulmonologist’s services, the usual standard-of-care outside testing, and a best effort to obtain enough tissue to screen my cancer cells against a panel of likely drugs for the new mutations that have shown up last year while on Lorlatinib. I ran the options by my local oncologist, and she noted that going there would allow to avoid the possibility of any delays or other snafus between the two big academic bureaucracies, and that the live tissue would make it to the lab in optimal condition. So, that’s what I did.
Dr. Qin at UMichigan conferred with Dr. Camidge about what drugs to try against my cancer cells. She also spoke directly with the UM interventional pulmonologist she works with all the time and told him exactly what was needed, which was the maximum number of biopsy needle passes obtainable. The procedure was scheduled for the following Monday, and I booked an inexpensive and very nice hotel on campus within walking distance of the hospital, after flying in from California. The biopsy went off without a hitch, obtaining eight needle passes. Laura Goo, the lead patient coordinator, picked me up at the hotel, waited there all through the procedure, and drove me back afterwards. Her colleague Bryce was present in the procedure room to take possession of the tissue, put it in the proper growth medium, and hand walked it to the lab for immediate processing. That attention to detail signals how precious our tissue is and how critical they consider time and proper handling.
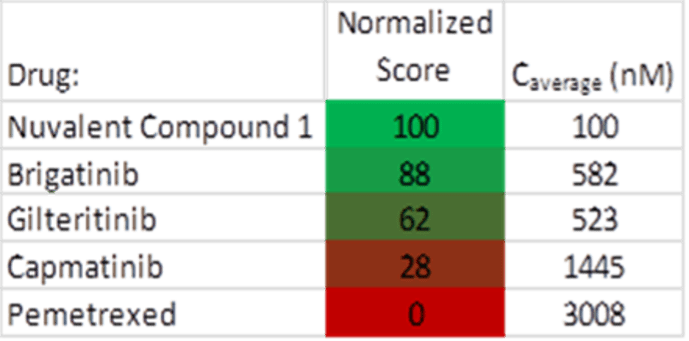
The next morning, I walked over for a lab tour and met the scientists I’ve been talking to for a few years. I looked at organoids through a microscope, small clusters of cancer cells grown from biopsy tissue or malignant fluid into clusters of a few hundred cells. The organoids are subsequently separated back into individual cells, which are deposited into an array of fluid-filled wells. Each well is then further filled with a solution containing a carefully calibrated concentration of the drug they want to test on the cancer cells. The array is placed in a machine that captures images of the process in each well over time. The results are studied for effectiveness, and the hopefully optimal drug for a patient’s cancer is thereby determined. This methodology is sometimes considered the holy grail of precision medicine. On my tissue they tested Brigatinib, Gilteritinib, Capmatinib (for possible MET mutation), Pemetrexed, and Nuvalent compound 1 (structurally similar to NVL-655).
The lab will also do their own genetic testing at a deeper level than the commercial services, which may identify other potential targets for treatment. While UM is not yet certified to provide formal diagnoses like Foundation Medicine, they can suggest treatments to my local oncologist and Dr. Camidge as a result of their process. Medicare paid for all the hospital bills, and, except for the travel expenses, the lab studies are all part of the ALK Research Initiative funded by Judith Tam and others, at no cost to the patient (although donations are appreciated).
While we have been promoting ALK tissue and fluid contributions to UM remotely via clinics with Material Transfer Agreements, the reality is that not many patients can pull that off with their existing care team. A further reality is that the UM ALK Initiative is not getting enough tissue to fully enable all their experiments. So, I am personally encouraging ALK patients to take advantage of this opportunity to test their own cancer against a panel of drugs and also donate tissue for research by going directly to UMichigan for their next biopsy. ALK Positive will soon be setting up a fund to help pay for travel expenses for qualified patients who need assistance and want to participate.
At the earliest opportunity (like now) you’ll need to register with the UM team well before going there via email at ALK-Initiative@med.umich.edu. After registering with UMichigan, should a likely tumor in excess of one centimeter eventually show up, you can confer with Dr. Angel Qin and team on whether you are a candidate for the screening. It’s then easy enough to explain to your oncologist that you want to get your biopsy done at UMichigan for their extended testing services, after having UM check on your insurance coverage. Your oncologist remains in charge of your care, gets the Foundation Medicine NGS test results as usual, and benefits from an additional level of information about what drugs may kill your cancer. There’s no work for your oncologist or clinic to do, other than electronically forward your medical records to UM.
My results were promising, with caveats. In particular, the Nuvalent compound 1 is not the exact one currently in clinical trials, but it has a similar structure. Nuvalent compound 1 and Brigatinib had the most effect on my cancer cells with UM confidence scores of 100 and 88 respectively (out of a possible 100). Gilteritinib had a modest effect, and there was very little effect from Capmatinib, zero from Pemetrexed. As I’m currently progressing on Lorlatinib and am waiting for an NVL-655 trial slot to open at UC Davis near me, it’s very good to know that second generation ALK TKI Brigatinib may also be an option.
So, I’m really glad I made the trip. The UMichigan team treated me like royalty, the right kind of surgeon did the biopsy, so they have some of my tissue for their research studies, and I have confidence (but not proof) that both NVL-655 and Brigatinib are viable options for me. I also got to spend inspiring time with brilliant researchers that are wholly devoted to finding better treatments for ALK patients. That alone was worth the trip.
https://www.rogelcancercenter.org/judith-tam-alk-lung-cancer-research-initiative
Author: Jeff Sturm, 9-year ALK survivor